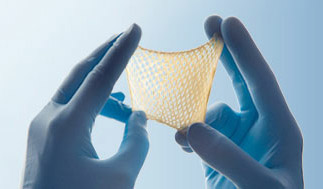
TheraSkin®
TheraSkin® is a biologically active, cryopreserved human skin allograft with both epidermis and dermis layers. Its cellular and extracellular composition provides a supply of growth factors, cytokines and collagen to promote wound healing.
Misonix, Inc.
Misonix, Inc. designs, develops and manufactures an array of therapeutic ultrasonic medical devices. Devices combine precision with power, utilizing state-of-the-art technology.Benefits
• Provides a broad spectrum of the major growth factors for wound healing
• Has the relevant, biological wound healing characteristics of human skin
• Fourteen types of human collagen necessary to promote healing
• Provides a significantly higher quantity of human collagen
• Twelve different growth factors
• Sixteen different cytokines
Warnings and Precautions
Do not use TheraSkin® after the expiration date indicated on the labeled unit carton. Follow all instructions to ensure the dermis, white side, is placed on the wound site.
Use aseptic techniques at all times. Keep frozen until preparing for implantation. Thaw each piece of TheraSkin® individually.
How Supplied/Sizing
Product features
Mode of Use/Application
Prepare the wound bed per protocol by removing all necrotic tissue, residual blood or exudate, and cytotoxic agents. Ensure there is no infection present. Visually inspect the outer packaging to ensure that it is intact and that its integrity has not been breached. If the outer packaging is damaged, the enclosed TheraSkin may be contaminated and should not be used.
Non Sterile Team Member: Open the SWAI box to expose the inner thermal container. Lift the lid of the thermal container and remove the TheraSkin envelope from the dry ice. Open the TheraSkin envelope and remove the inner foil pouch. Inspect inner pouch for damage. Use scissors to open the foil pouch and remove the plastic pouch containing the TheraSkin. Pour 500-1000ml of room temperature sterile saline/water solution into the first sterile basin. Do not exceed 42C (108F). Continuing to use clean handling techniques, peel open the plastic pouch and present to the Sterile Team Member.
Sterile Team Member: Remove TheraSkin from the plastic pouch and place it into the first sterile basin. Allow the TheraSkin to thaw completely. It will be readily pliable when thawed, at which time you may unfold the mesh pack. Do not exceed 42C, as it may damage the TheraSkin. Fill second basin with 500-1000 ml room temperature sterile saline/water solution, not exceeding 42C. Remove TheraSkin mesh pack from first basin and carefully lift TheraSkin away from mesh lining. Place TheraSkin only (without mesh lining) in the second basin and soak for a minimum of five minutes. TheraSkin is now ready for application. Keep TheraSkin completely submerged in sterile saline water solution until it is time for application. Do not allow TheraSkin to dry before application.
Placement
Remove TheraSkin from the basin using sterile pick-up. Apply TheraSkin over the wound bed using aseptic techniques, with the epidermis (pigmented side) away from the wound bed (facing up) and the dermis (collagen, white side) in contact with the wound bed (facing down). When applying TheraSkin, it is beneficial to have as much TheraSkin in contact with the wound bed as possible. The edges of the TheraSkin can be gently pushed toward the middle of the wound to minimize open mesh areas, without doubling the layers of the TheraSkin.
Fixation
Leave a 2-5 mm rim of overlapping TheraSkin around the wound wherever possible, trim away the excess TheraSkin. For large wounds, consider placing a suture in the center of the wound, to ensure that the TheraSkin stays in close contact with the wound bed. Secure TheraSkin in place, using sutures, staples, steric-strips or Dermabond as needed.
Top Dressing Application
TheraGauze+FN is recommended as the cover dressing for TheraSkin. Remove TheraGauze+FN from foil package. Place TheraGauze+FN dressing over the wound. If wound is dry and dessication may be a factor; a second piece of TheraGauze+FN can layered on top of TheraSkin. For a small wound, TheraGauze+FN may be folded over to obtain two layers. Place a gauze pad on top of TheraGauze+FN, and secure dressings in place with gauze wrap. This dressing should be left in place for 5-7 days to maximize effectiveness.
Dressing Change
If TheraGauze+FN is dry in appearance during the first dressing change, rehydrate by applying sterile solution to the TheraGauze+FN and wait 1-3 minutes before attempting to remove. If/once the TheraGauze+FN is moist, grasp the edge of the dressing and slowly lift from the skin and wound site. Inspect TheraSkin incorporation into the wound bed. Re-dress wound if necessary.
Technical Specifications
Tissue is processed from donated human skin tissue and is provide by the Skin and Wound Allograft Institute (SWAI), a wholly owned subsidiary of LifeNet Health, Inc.
All TheraSkin® has been recovered, processed, stored, and distributed according to the Standards for Tissue Banking set forth by the American Association of Tissue Banks and FDA Current Good Tissue Practices.
TheraSkin® has been determined to be suitable for transplantation. Only licensed clinical professionals can implant TheraSkin®.
All TheraSkin® and TheraSkin® donors undergo strict screening and testing protocols for potential disease and infection transmission in compliance with the American Association of Tissue Banks and Federal Regulations.
The cutaneous reservoir for HIV, namely the Langerhans cell, does not survive the cryopreservation process. No case of HIV transmission by properly cryopreserved and banked human allograft skin from properly screened donors has ever been reported. (Spence, R. and Wong, L.: The Enhancement of Wound Healing with Human Skin Allograft. Surgical Clinics of North America 1997;77.3:731-745.)
TheraSkin has been determined suitable for use [graft] to repair skin over any wound including those with exposed muscle, tendon, bone and joint capsule.
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More












Follow WoundSource
Tweets by WoundSource