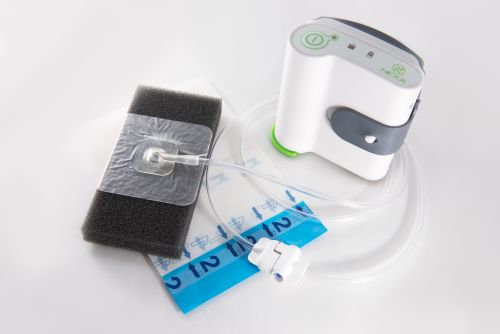
NEXA™ NPWT System
NEXA™ NPWT System is a multi-week, multi-patient, disposable NPWT system that will operate for 56 days. Lightweight and portable with carrying case and rechargeable battery. Flexible fluid container pouch holds 120ml of exudate.
Advanced Oxygen Therapy Inc. (AOTI)
Advanced Oxygen Therapy, Inc. (AOTI) offers the only multi-modality topical oxygen wound therapy (TWO2) on the market.Benefits
• Disposable product
• Can be used for a total of 56 consecutive or non-consecutive therapy days
• One device may be used on multiple patients
• Visual-only alerts for reduced "alarm anxiety"
• Unique hydrodynamic fluid pump continues to remove exudate even in the presence of a minor dressing leak, thereby reducing the potential for skin maceration
• Low daily device cost
Indications
The NEXA™ NPWT system is intended for patients who may benefit from the application of negative
pressure to the wound to promote wound healing through the removal of excess exudates, infectious
material and tissue debris. It is intended for use in long-term care and acute settings only when
prescribed by a health care professional. Appropriate wound types to include chronic wounds, pressure ulcers, diabetic foot ulcers, venous leg ulcers, acute wounds, surgical incisional wounds, subacute and dehisced wounds, partial-thickness burns, flaps and grafts.
Contraindications
The device is not recommended for treatment of the following conditions: presence of necrotic tissue; malignancy in wound; untreated osteomyelitis; untreated malnutrition; exposed arteries, veins, nerves, or organs; use over anastomotic sites; non-enteric and unexplored fistulas; necrotic tissue with eschar.
Warnings and Precautions
PRECAUTIONS:
• Patients on anticoagulation medicine or who have active bleeding or who have difficult wound hemostasis should be treated with caution. These patients are at an increased risk for bleeding and bleeding complications and should be treated and monitored by properly trained medical caregivers in a controlled setting.
• Wounds that are in close proximity to blood vessels, organs, muscle, and fascia: All blood vessels, organs, muscles, and fascia that are in close proximity to the wound site and/or are exposed and /or are near the skin surface should be properly protected prior to initiating therapy. Patient with infections in the wound and or other parts of the body have to receive proper systemic treatment.
• Irradiated vessels and tissue. These patients are at an increased risk for bleeding and bleeding complications and should be treated and monitored by properly trained medical caregivers in a controlled setting.
• Bony fragments. Sharp edges from bony fragment may puncture blood vessels, organs, muscles, and fascia and may lead to bleeding. Proper care should be taken to cover the bony fragments and protect the wound area and other areas from bleeding.
• Check wound dressing periodically to ensure there is no build up of wound fluid or evidence of any bleeding.
• Dressings should be routinely changed every 48 - 72 hours but no less than 3 times a week or when the wound shows signs of infection.
• Do NOT continue using the system if a hematoma is observed when changing the dressing.
• Spinal cord injury: In the event a patient experiences autonomic dysreflexia, discontinue the use of the NEXA system and seek advice.
• Bradycardia: The NEXA system must not be placed in proximity to the vagus nerve.
• Enteric fistulas: The NEXA system is not recommended if enteric fistula effluent management is the sole purpose of management.
• Consider using a skin preparation product to protect the periwound skin. If any signs of irritation appear, cease use of NEXA.
• Circumferential dressing application: Do not use circumferential dressings unless required to maintain a seal in the presence of anasarca or excessively weeping extremities. It is recommended to use smaller strips to obtain a seal rather than one large drape. Do not stretch the drape upon securement.
• Care must be taken to assess the wound for anastomotic sites. If present, use a non-adherent interface layer between the wound and the foam.
• To prevent tissue ingrowth, consider using an interface layer between the foam and wound. Also consider selecting a lower pressure dressing.
Precautions should be taken for patients with the following conditions:
• It is important that a doctor, nurse or other qualified health care provider evaluates the patient to ensure that the use of this device is an appropriate therapy.
• Prior to issuing the system, the patient should be assessed for their knowledge, skill level and amount of training required enabling the patient to use the system themselves.
• To reduce the risk of transmission of blood-borne pathogens, regardless of the diagnosis or presumed infection status, all users should take suitable precautions as defined in locally applicable standard operating procedures for infection control.
The following Warning statements describe the potential for serious consequences to the patient, such as death, injury, or adverse reactions.
Failure to read and follow all instructions in this manual prior to use may result in death or injury of the patient.
• Do not use any Power Supply to re-charge the device other than that supplied (part no.:414-02-015)
• The socket in which the Power Supply is connected must be accessible at all times.
• Only use dressings and accessories that are approved for use with the NEXA device.
• Physician should consider the patient’s size and weight when prescribing this device.
• The device is not safe for use with an MRI and must be disconnected from the patient prior to MRI.
• Do not use the device in a hyperbaric chamber or in the presence of flammable gases. The patient’s dressing may remain in place when disconnected from the unit.
• To prevent unintentional gauze/foam retention, all dressings should be carefully removed from the wound and the entire wound bed. Upon removal of the dressings, the wound bed should be cleaned in accordance with standard wound care practices (or facility guidelines), prior to the application of new sterile dressing.
• Ensure that there are no pockets left in the wound or wounds after application of the dressings.
• Infected wounds must be inspected more frequently for signs of increased infection or sepsis. • Patients who do not have adequate hemostasis, and who are currently taking anticoagulation or platelet aggregation inhibitor therapy, have an increased risk of bleeding with or without the NPWT device.
• Defibrillation: Remove the NEXA Dressing if defibrillation is required in the area of dressing placement. The dressing may inhibit transmission of electrical energy and/or patient resuscitation.
• All arteries, veins, tendons, ligaments, nerves, and organs must be covered completely prior to application of the device.
• Only use with caution on any patient with increased risk of bleeding due to the presence of weakened or friable blood vessels or organs, conditions when suture of the blood vessels has taken place or when there is (localized to the wound) any infection, trauma, or radiation, as if not controlled well, could potentially be fatal.
• Infected tissue such as blood vessels may have a weakened structure and have to be treated with care. Infected blood vessels may bleed more readily than normal blood vessels.
• There is a risk of strangulation or asphyxiation from tubing, cables and the carry case strap. Retain excess tubing to patients body using supplied drape.
• In the event of a device malfunction return the equipment to the authorized service center.
• There are no serviceable parts inside the therapy unit therefore do not open the device.
• No modification of the device is allowed!
• The device must never be used to remove explosive gases and flammable or corrosive fluids.
• The device must not be operated in damp rooms or when taking a bath or shower. Avoid moisture on plug and switches.
• Never plunge the device into water or liquids, not even when it is switched off.
• The unit must not be operated in splash water range, near sources of steam such as kettles or in locations where there is a danger of explosion.
• EMC Statement: Although the device is compliant with the current EMC regulations applicable, the device may be susceptible to EMC radiation and action should be taken to avoid exposure.
• The NEXA NPWT System is a medical device, not a toy. Keep away from children, pets and pests as they can damage the NEXA Device,
NEXA Dressing and Fluid Container Pack and potentially affect their performance. Keep the system free from lint and dust as they may cause damage and affect performance.
• Keep the system away from sources of heat such as heaters, fireplaces or direct sunlight.
• To ensure the system is working correctly, the dressing and device should be checked regularly throughout the day to check for fluid mobility in the tubing and that the green led is lit.
• The NEXA device does not have any audible or visual alarms to alert the user to a leak or blockage in the system. Please refer to the troubleshooting section to determine how a leak or blockage may be detected and resolved.
• The foam and drape dressing components that come into patient contact may cause an allergic reaction. Ensure the foam does not overlap on to healthy skin and always monitor the skin for any signs of reaction.
• Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the member state in which the user and/or patient is established.
Storage Requirements
Temperature Range:
NEXA Device and Fluid Container Pack: -25°C-70°C (-13°F-158°F)
NEXA Dressing: 10°C-27°C (50°F-80°F)
Relative humidity range: 0-93% non-condensing
How Supplied/Sizing
Product features
Other features
Recommended Use
Acute Wounds
Burns
Chronic Wounds
Dehisced Wounds
Diabetic Foot
Pressure Ulcers
Surgical Wounds
Venous Ulcers
Mode of Use/Application
The NEXA™ NPWT System is an integrated negative pressure wound management system for use in
acute and extended care settings. The system applies a negative pressure to a sealed wound dressing
and promotes wound healing through the removal of exudates into a disposable fluid container.
Removal & Change Frequency
Dressings should be changed every 48-72 hours but no less than 3 times per week.
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More












Follow WoundSource
Tweets by WoundSource